What is dissolved oxygen?
Dissolved oxygen (DO) refers to molecular oxygen dissolved in water. The unit is mg/L, which means how many milligrams oxygen in per liter of water. The oxygen levels can reflect the self-purification ability in the water. In an environment with high DO, it is beneficial to degrade various pollutants and make the water body be purified faster. Conversely, in a low DO environment, pollutants in water degrade more slowly.
DO levels are affected by water temperature, air pressure and salinity. Decreases with increasing water temperature, the higher the temperature the lower the oxygen content. The dissolved oxygen value is a basis for studying the self-purification ability of water. In a flowing river, water samples are taken from different sections to determine the oxygen content. It is possible to understand the self-purification of the river at different locations. Generally, the oxygen content in water is at least 4mg/L, so the water quality is better. In the wastewater treatment process, the oxygen content value is also an important indicator.
What is dissolved oxygen?
The main factors affecting the dissolved oxygen level in water are water temperature, salinity, and atmospheric pressure.
1. How does temperature affect dissolved oxygen
As the temperature increases, the oxygen content gradually decreases. In freshwater, dissolved oxygen mainly comes from two parts: one is the oxygen released by plant photosynthesis, accounting for about 60%. Another part is that the oxygen in the air dissolves in water, accounting for about 40%. When the temperature rises, the gaps between the water molecules become smaller, the oxygen in these gaps is squeezed out, and the oxygen content drops. Usually, the DO concentration in the water is the lowest in summer.
| The saturated dissolved oxygen concentration in water and its corresponding temperature | |||||
|---|---|---|---|---|---|
| Temperature | DO (mg/L) | Temperature | DO (mg/L) | ||
| ℉ | ℃ | ℉ | ℃ | ||
| 32 | 0 | 14.6 | 74 | 23.3 | 8.5 |
| 34 | 1.1 | 14.1 | 76 | 23.4 | 8.3 |
| 36 | 2.2 | 13.7 | 78 | 25.6 | 8.2 |
| 38 | 3.3 | 13.3 | 80 | 26.7 | 8.0 |
| 40 | 4.4 | 12.9 | 82 | 27.8 | 7.8 |
| 42 | 5.6 | 12.2 | 84 | 28.9 | 7.7 |
| 44 | 6.7 | 11.9 | 86 | 30 | 7.5 |
| 46 | 7.8 | 11.6 | 88 | 31.1 | 7.4 |
| 48 | 8.9 | 11.3 | 90 | 32.2 | 7.3 |
| 50 | 10 | 11.0 | 92 | 33.3 | 7.1 |
| 52 | 11.1 | 10.7 | 94 | 34.4 | 7.0 |
| 54 | 12.2 | 10.4 | 96 | 35.6 | 6.9 |
| 56 | 13.3 | 10.2 | 98 | 36.7 | 6.8 |
| 58 | 14.4 | 9.9 | 100 | 37.8 | 6.6 |
| 60 | 15.6 | 9.7 | 102 | 38.9 | 6.5 |
| 62 | 16.7 | 9.5 | 104 | 40 | 6.4 |
| 64 | 17.8 | 9.3 | 106 | 41.1 | 6.3 |
| 66 | 18.9 | 9.1 | 108 | 42.2 | 6.2 |
| 68 | 20 | 8.9 | 110 | 43.3 | 6.1 |
| 70 | 21.1 | 8.7 | 112 | 44.4 | 6.0 |
| 72 | 22.2 | 114 | 45.6 | 5.9 | |
Everyone who has seen boiling water knows that air bubbles form on the sides and bottom of the pot during the heating process. Bubble number and size increase with temperature. These are air bubbles that have been dissolved in water. We can imagine water as a homogeneous medium. It has many holes scattered throughout its volume. The air pressure above the water’s surface will fill these holes with air. So when the temperature rises, this air is forced to separate from the water molecules. These bubbles also contain oxygen, so the oxygen content will decrease.
2. How does salinity affect dissolved oxygen
As salinity increases, oxygen levels also decrease. Because when the salinity in the water increases, these salt molecules occupy the spaces between the water molecules. thereby reducing the oxygen content. For example, in a bottle filled with stones, pour some fine sand, then there will be less air in the bottle. Because the fine sand fills the gaps between the stones.
| Relationship between DO, temperature and salinity in water at standard atmospheric pressure (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (℃) | Salinity | ||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | |
| 20 | 9.1 | 8.8 | 8.7 | 8.3 | 8.1 | 7.9 | 7.7 | 7.4 | 7.2 |
| 22 | 8.7 | 8.5 | 8.2 | 8 | 7.8 | 7.6 | 7.3 | 7.1 | 6.9 |
| 24 | 8.4 | 8.1 | 7.9 | 7.7 | 7.5 | 7.3 | 7.1 | 6.9 | 6.7 |
| 26 | 8.1 | 7.8 | 7.6 | 7.4 | 7.2 | 7 | 6.8 | 6.6 | 6.4 |
| 28 | 7.8 | 7.6 | 7.4 | 7.2 | 7 | 6.8 | 6.6 | 6.4 | 6.2 |
| 30 | 7.5 | 7.3 | 7.1 | 6.9 | 6.7 | 6.5 | 6.3 | 6.2 | 6 |
| 32 | 7.3 | 7.1 | 6.8 | 6.7 | 6.5 | 6.3 | 6.1 | 6 | 5.8 |
| 34 | 7.0 | 6.8 | 6.6 | 6.4 | 6.3 | 6.1 | 5.9 | 5.8 | 5.6 |
| 36 | 6.8 | 6.6 | 6.4 | 6.2 | 6.1 | 5.8 | 5.7 | 5.6 | 5.4 |
| 38 | 6.5 | 6.3 | 6.2 | 6 | 5.8 | 5.7 | 5.5 | 5.4 | 5.2 |
| 40 | 6.3 | 6.2 | 5.9 | 5.8 | 5.6 | 5.7 | 5.3 | 5.2 | 5 |
3. How Air Pressure Affects Dissolved Oxygen
The greater the air pressure, the more oxygen molecules can be pressed into the water, and the more oxygen there is in the water. The pressure from above enables the water to hold more oxygen molecules. Conversely, the smaller the air pressure, the easier it is for the oxygen in the water to overflow. Generally, the dissolved oxygen is very low when the air pressure is low, such as before it rains, especially before the heavy rain in summer.
Why is dissolved oxygen important?
Just like humans use their lungs to absorb oxygen from the air, organisms in the water also need to absorb oxygen from the water to survive. Dissolved oxygen is very important for aquatic organisms. Fish, invertebrates, crabs and other underwater animals use their gills to get oxygen from the water. Algae and other phytoplankton need to consume oxygen for respiration when there is no light for photosynthesis. When microorganisms such as bacteria and fungi decompose organic substances in water, they also need to consume oxygen. When the oxygen in the water is sufficient, the animals and plants in the water can grow healthily.
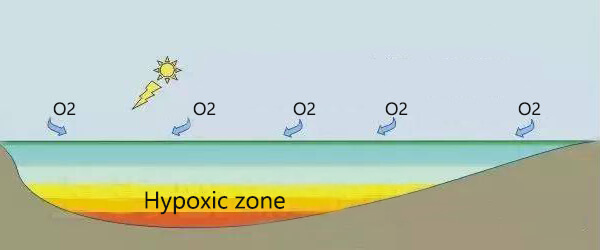
In ponds or rivers, oxygen levels fluctuate periodically and seasonally for natural ecological reasons. As the dissolved oxygen decreases, the fish will move closer to the water’s surface and even stick their heads out of the water. If the oxygen deficiency is severe, the fish will get sick or even die. Sufficient oxygen in the water can inhibit toxic substances and effectively degrade toxic substances. When oxygen is insufficient, ammonia and hydrogen sulfide are difficult to decompose and transform, which will affect water quality and biological health.
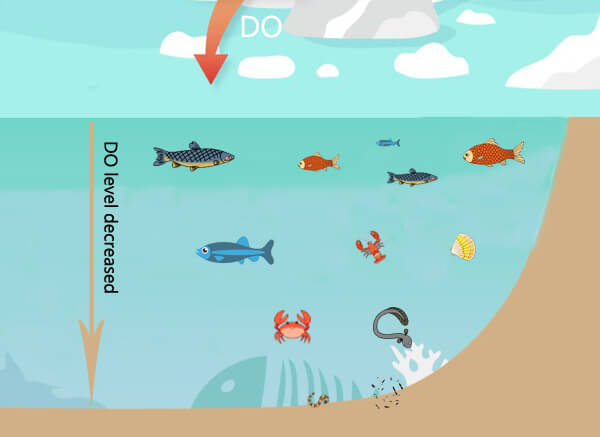
As the water gets deeper, it contains less and less oxygen. This also creates species diversity. Different organisms are active in the water layer that suits them according to their own needs. Organisms that live in deep water, such as crabs, oysters, etc., need less oxygen (1-6 mg/L), but fish that live in shallow water needs more oxygen (4-15 mg/L)⁵.
How to measure dissolved oxygen?
The titration method is to add a certain concentration standard solution dropwise to the tested solution (or drop the tested solution to the standard solution) until the two solutions react completely. Then measure the consumed volume and concentration of the standard solution to calculate the measured substance.

Adding manganese peroxide solution and sodium hydroxide solution to the measurement sample water will generate manganese hydroxide precipitate. Manganese hydroxide is chemically very unstable and reacts with dissolved oxygen in water to form a brown precipitate. In the presence of iodide ions (I-), concentrated sulfuric acid was added to promote the complete reaction of the brown precipitate. Slowly release iodine. The more dissolved oxygen in the water, the more iodine will be released and the darker the solution will be. Then, the released iodine was titrated with sodium thiosulfate and its amount was determined. Calculate the oxygen content in the water.
Titration is the earliest method used to measure dissolved oxygen in water, and it is suitable for measuring water samples with dissolved oxygen greater than 0.2mg/L. This method can be used to determine the oxygen content of pure water, but it is not suitable for the determination of dissolved oxygen in industrial wastewater or sewage treatment plants. Especially when the water contains some reducing substances such as sodium nitrite, sulfide, thiourea or humic acid, it will interfere with the measurement data, resulting in large errors in the measurement data.
Fluorescence method
Fluorescence dissolved oxygen method uses the fluorescence quenching principle to measure the oxygen content. When the blue light is irradiated on the fluorescent substance, the fluorescent substance is excited and emits red light. Since oxygen molecules can take away energy (quenching effect), the time and intensity of the red light are inversely proportional to the concentration of oxygen molecules. By measuring the phase difference between the excited red light and the reference light, and comparing it with the internal calibration value, the oxygen content can be calculated. The most popular dissolved oxygen meter is based on the fluorescence method principle.
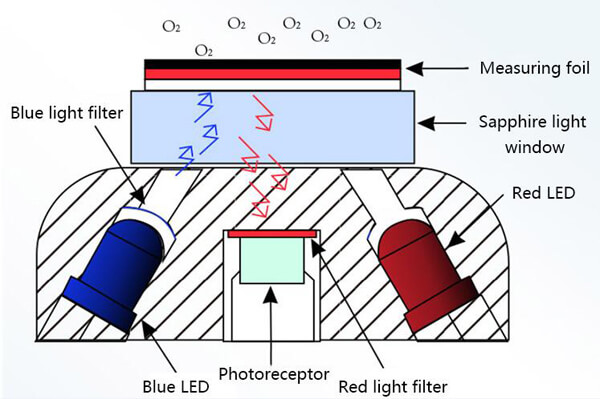
Compared with other methods, the fluorescent dissolved oxygen sensor is easy to use and the measured data is more accurate. You only need to insert the electrode into the solution to be tested and wait for 1-2 seconds, then you can get the measured value from the digital display. The whole process does not consume oxygen in the water. Widely used in urban sewage and industrial wastewater treatment plant monitoring.
Diaphragm electrode method
The electrode used in the diaphragm electrode method consists of a small chamber. The chamber contains two metal electrodes and is filled with electrolytes, and the small chamber is closed by a selective membrane. Water and soluble ions cannot pass through the membrane, but oxygen and hydrophilic ions can. Dissolved oxygen measurements were performed by immersing the electrode in water.
The potential difference between the electrodes is caused by a battery or an applied voltage. This potential difference causes metal ions to enter solution at the anode, while oxygen permeating the membrane is reduced at the cathode. The resulting electrical current is proportional to the rate of oxygen transport and therefore proportional to the partial pressure of oxygen in the water. Temperature compensation is necessary because the permeability of the membrane changes with temperature. You can use math, or you can use regulators. Or compensation can be achieved by installing a thermal sensor in the circuit. Some instruments can also compensate for changes in oxygen solubility at different temperatures.
This measurement method is suitable for natural water, sewage and salt water. If you want to measure brackish water such as seawater or harbor water, you must correct the salt content. Compared with titration, it can measure water with high color and high turbidity. It is even used for the determination of iron-containing substances and substances that can interact with iodine. If there are gas molecules in the water sample that can diffuse and pass through the membrane, it will interfere with the measured current. Solvents, oils, sulfides, carbonates and algae in the water can cause membrane plugging, membrane damage or electrode corrosion.
Colorimetric Method
There are two variations in measuring oxygen content by colorimetry. Indigo Carmine and Rhodazine D method. Both methods use colorimetric reagents. They react and change color when reacting with oxygen in the water. These interactions are based on oxidation reactions, and the color change is proportional to the oxygen content. Colorimetric results can be compared with a spectrophotometer, colorimeter, or other comparator. Results are more accurate using a spectrophotometer or colorimeter. Using a color patch comparator is fast and cheap, but it may cause some inaccuracies due to the impersonal nature of the human eye.
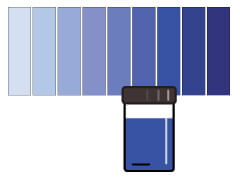
According to the indigo carmine method, the darker the blue color, the higher the dissolved oxygen concentration.
The indigo carmine method measures oxygen levels in the range 0.2 and 15 ppm (mg/L). This method produces a blue color whose intensity is proportional to the dissolved oxygen concentration. Iron, ferrous, nitrite and sodium bisulfite can interfere with this method. In addition, the reagent should be kept away from strong light, as prolonged exposure will reduce the quality of indigo carmine. However, the method is not affected by temperature, salinity or dissolved gases. Low range tests are time dependent and should be analyzed within 30 seconds, while high range tests require 2 minutes of processing time.
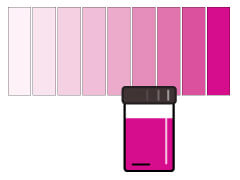
The rhodazine D method produces a rose or pink color.
The Rhodazine D method can measure very low oxygen content. Rhodazine D reagent reacts with dissolved oxygen in parts per billion (ppb) to form a deep rose colored solution. The colorimetric method is not affected by salinity or sulfides in the water. However, oxidizing agents (chlorine, copper, and iron) can interfere with DO readings. Other error causes are polysulfides, boron and hydrogen peroxide. Sample color and turbidity can affect accuracy. This method is relatively time demanding, and the water sample must be analyzed within 30 seconds after adding the reagent.
How to increase dissolved oxygen in water?
When the oxygen content in the water is insufficient, it will have adverse effects on aquatic animals. Water quality deteriorates, thereby affecting aquatic life. Harmful to its growth, reproduction and even survival. In mild cases, physical fitness decreases and the growth rate slows down, and in severe cases, a large number of deaths are caused. Therefore, in the aquaculture industry, it is very important to monitor the oxygen content and increase the oxygen in time.
1. Biological oxygenation
Aquatic plants can produce oxygen through photosynthesis and add it directly to the water. This is the most cost-effective and scientific method. It uses natural methods to increase oxygen in the pond and will not affect the organisms. Chloroplasts in plant cells use light energy to convert carbon dioxide and water into glucose and oxygen. So, increase the oxygen by adding algae plants in the water. Regularly add nutrients needed for algae reproduction to the water. Promote algae growth and reproduction. Using this method to increase DO requires consideration of adequate sunlight and nutrients. Because when there is sufficient light, aquatic plants can only carry out photosynthesis. At night or when there is no light, plants perform respiration, which consumes oxygen in the water.
2. Physical oxygenation
Use machinery and equipment, such as aerators, to continuously pump air into the water. Increase the contact area between water and air, allow air to better combine with water, and increase the oxygen content in water. Or directly transport the air to the bottom of the pool to increase the contact area and contact time between the air and the water body. Let it dissolve more. Increase freshwater sources to increase dissolved oxygen in the water. On a sunny afternoon, turn on the aerator appropriately to let the water flow. Stir some oxygen-consuming substances at the bottom to the middle and upper layers, let them oxidize and decompose, and reduce the oxygen consumption at the bottom.
3. Chemical oxygenation
Sprinkle oxygen-increasing tablets, hydrogen peroxide, and some surfactants to the pond to increase the oxygen content in the water in a short time. This method is suitable for use in emergencies when the water is severely hypoxic and there is no aerator.