Table of Contents Water quality parameters are indicators used to assess the condition and trends of aquatic environments. Monitoring is
ORP Sensor
RS-ORP-N01-3-EX ORP sensor is used to measure the oxidation-reduction potential in the water. ORP value is an important indicator of water quality. The measuring range of this ORP sensor is -1999~1999mV and the accuracy is ±1mV, which can meet your needs for any monitoring environment. Factory-direct sales, cheap price.
- Model: RS-ORP-N01-3-EX
- MOQ: 1 PCS
- Delivery date: within 24 hours
- Price: USD 73.40
ORP Sensor Description
RS-ORP-N01-3-EX ORP sensor is a device for measuring the oxidation-reduction potential of water. ORP electrode made of high-purity platinum has strong resistance to acid and alkali and oxidation, high measurement accuracy and good stability. The electrode can be automatically compensated according to the temperature. It is suitable for online monitoring of oxidation-reduction potential of cyanide and chromium-containing wastewater.
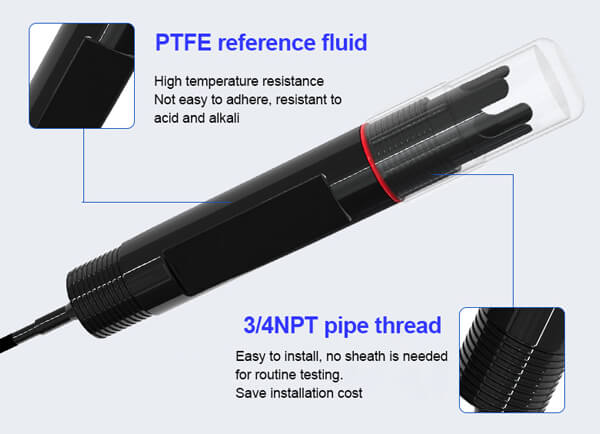
OPR Meter Parameters
Power supply: DC 7~30V
ORP measuring range: -1999~1999mV
Resolution: 1mV
ORP measurement error: ±5mV
Output mode: RS485
Equipment working conditions Ambient temperature: 0-60℃
Relative humidity: <85%
Applicable temperature of electrode: 0~60℃
Electrode withstand voltage: 0.6MPa
Electrode wire length: default 5m (10m, 15m, 20m can be customized)
Electrode life cycle: 1 year
ORP Sensor Application
Wastewater
ORP sensors can be used to treat industrial wastewater. The redox system used in water treatment is mainly the reduction of chromic acid and the oxidation of cyanide.
Pure water
Redox electrodes can measure the disinfection effect of swimming pool water, mineral water, and tap water. Because the bactericidal efficiency of coliform bacteria in water is related to the oxidation-reduction potential.
Breeding industry
ORP sensor can reflect the concentration of certain inorganic substances in the water, the state of aquatic organisms, and the oxygen content in the water, and reflect the macro-oxidation and reduction properties of water quality. It is a comprehensive indicator for judging water quality.
ORP Sensor FAQS
ORP is the abbreviation of Oxidation-Reduction Potential in English, which represents the oxidation-reduction potential of the solution. ORP value is a measurement index of the redox ability of aqueous solution, and its unit is mv. It is composed of ORP composite electrode and mv meter. ORP electrode is an electrode that can absorb or release electrons on the surface of its sensitive layer. The sensitive layer is an inert metal, usually made of platinum and gold. The reference electrode is the same silver/silver chloride electrode as the pH electrode.
ORP stands for the oxidation-reduction potential of a solution, which is an important indicator of water quality. It cannot independently judge the quality of water, but it can integrate other water quality indicators to reflect the ecological environment in the aquarium system. The higher the oxidation-reduction potential, the stronger the oxidation, and the lower the potential, the weaker the oxidation. A positive potential indicates that the solution shows a certain degree of oxidation, and a negative potential indicates that the solution shows reduction.
The actual meaning of orp measurement has the following situations:
1. Indirectly reflects the degree of concentration accumulation of nitric acid and other substances in the water.
2. Monitor the oxidation efficiency of microorganisms in filtration.
3. Reflect the concentration of certain inorganic substances in the water and the state of aquatic organisms.
ORP value is affected by PH value, but ORP cannot reflect the specific value of PH.
◆ The ORP sensor itself generally does not require routine maintenance. When there is an obvious failure, please do not open it and repair it yourself, and contact us as soon as possible!
◆ Under normal circumstances, the ORP electrode does not need to be calibrated, just use it directly. When in doubt about the quality and test results of the ORP electrode, you can use the ORP standard solution to check the electrode potential to determine whether the ORP electrode meets the measurement requirements, to recalibrate the electrode or replace with a new ORP electrode, to calibrate or check the frequency of the measurement electrode, Depends on different application conditions (the degree of dirt in the application, the deposition of chemical substances, etc.).
◆ There is an appropriate amount of soaking solution in the protective bottle at the front of the electrode, and the electrode tip is soaked in it to keep the platinum sheet and the liquid junction activated. When measuring, unscrew the cap, pull out the electrode, and wash it with pure water before use.
◆ Preparation of electrode soaking solution: Dissolve 25 g of analytically pure potassium chloride in 100 ml of pure water to prepare a 3.3M potassium chloride solution.
◆Before measurement, the bubbles in the electrode glass bulb should be shaken off, otherwise it will affect the measurement. During measurement, the electrode should be agitated in the measured solution and placed still to speed up the response.
◆ Use deionized water to clean the electrodes before and after the measurement to ensure accuracy.
◆ The ORP electrode will become passivated after long-term use. The phenomenon is that the sensitivity gradient is reduced, the response is slow, and the reading is inaccurate. At this time, the platinum sheet at the bottom of the electrode can be soaked in 0.1M dilute hydrochloric acid for 24 hours (0.1M dilute hydrochloric acid preparation: 9 Dilute one milliliter of hydrochloric acid with distilled water to 1000 milliliters), and then soak it in 3.3M potassium chloride solution for 24 hours to restore its performance.
◆ Electrode contamination or blockage of the liquid junction will also passivate the electrode. At this time, it should be cleaned with an appropriate solution according to the nature of the pollutant. See the table below for details (for reference).
Contaminants-cleaners
Inorganic metal oxide-less than 1M dilute acid
Organic oils and fats-thin detergent (weak alkaline)
Resin macromolecules-alcohol, acetone, ether
Protein blood precipitate——acid enzyme solution
Pigment substances——dilute bleaching solution, hydrogen peroxide
If the platinum electrode is seriously polluted and an oxide film is formed, apply toothpaste on the surface of the platinum, and then gently scrub it to restore the platinum’s luster.
◆ The electrode life cycle is about one year, and the new electrode should be replaced in time after aging.
Other Water Quality Sensors
Related Blogs
Table of Contents In wastewater treatment projects, it is essential to monitor water quality at every stage to ensure that
Table of Contents What do pH Mean in Water? The water pH is an important indicator of its acidity or
Table of Contents Everyone knows that the choice of water quality sensors is very important. In water measurement, the ph
Table of Contents Water is the source of life, integral to every aspect of human production and daily living. In
Table of Contents Have you or your friend experienced a broken water pipe, water accumulation in the kitchen or bathroom?
Table of Contents What is IoT? IoT is the “Internet of things“. It is an extended and expanded network based
Table of Contents What is a water level sensor? The water level sensor is a device that measures the liquid
Electronic water level measurement gauge is a new type of water level measurement sensor, which is composed of a PCB










