In daily life, industrial production, and laboratory research, the terms “distilled water” and “purified water” are often mentioned. Many people mistakenly assume they are the same. However, the two differ significantly in terms of production processes, water quality, and application scenarios. This article will provide you with a comprehensive comparison of distilled water and purified water, helping you make a scientifically informed choice based on your specific needs.
What is distilled water?
Distilled water is a type of purified water obtained through the process of distillation. This method involves heating water until it evaporates and then condensing the steam back into liquid form. Through this process, inorganic salts, heavy metals, bacteria, viruses, and most organic substances are effectively removed, resulting in high-purity water that is virtually free of impurities.
| Quality indicators of distilled water | ||
|---|---|---|
| Indicators | Standard value of distilled water | Description |
| Conductivity | ≤1μS/cm (25℃) | The higher the purity, the lower the conductivity |
| PH value | 5.0-7.0 (may be slightly acidic after exposure to air) | Slightly acidic due to absorption of CO₂ |
| Total dissolved solids (TDS) | <1mg/L | Almost no detectable impurities |
| Microorganisms | None | Killed by high-temperature steam process |
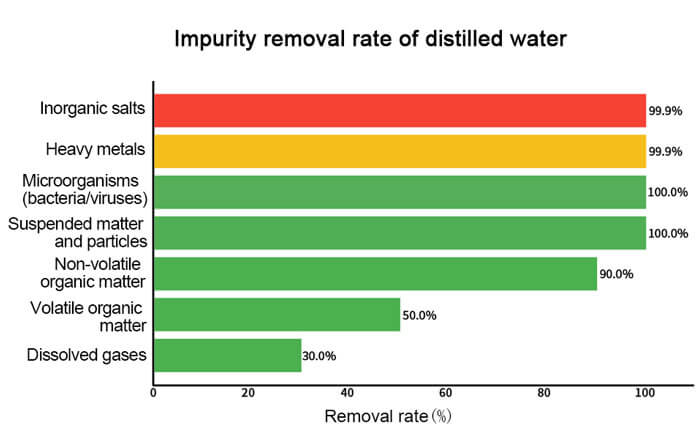
Distilled water is renowned for its nearly perfect purity. The distillation process removes almost all impurities and minerals, resulting in water with a pH value close to neutral and free from any additives. This high level of purity makes distilled water the preferred choice for laboratories, medical equipment, and certain specialized industrial applications. Although purified water is more commonly consumed as drinking water, distilled water is also safe for human consumption.
What is purified water?
Purified water refers to water that has been treated to remove most dissolved inorganic salts, organic compounds, microorganisms, and particulate matter. The purification process typically involves multiple advanced treatment methods, such as reverse osmosis, activated carbon filtration, ion exchange, and UV disinfection.
| Quality indicators of purified water | |||
|---|---|---|---|
| Item name | Standard value/Limit | Unit | Description |
| Conductivity | ≤10 | μS/cm (25℃) | Higher values reflect more dissolved ions |
| PH value | 6.5-7.5 | None | Close to neutral, avoid corrosion or precipitation problems |
| Total dissolved solids (TDS) | ≤10 | mg/L | Total of soluble salts and inorganic substances |
| Hardness (as CaCO₃) | ≤5 | mg/L | Reflects the content of calcium and magnesium ions |
| Chloride ion (Cl⁻) | ≤0.5 | mg/L | Exceeding the standard and prone to corrosion |
| Total bacteria | ≤100 | CFU/mL | Drinking water requirements, industrial water can be more stringent |
| Coliform bacteria | Not detectable | — | Drinking water standards |
| Total organic carbon (TOC) | ≤0.5-1.0 (industry differences) | mg/L | Reflects the level of organic pollutants in water |
| Color | ≤5 | Degrees | Generally close to colorless |
| Turbidity | ≤0.5 | NTU | The lower the clearer |
| Dissolved oxygen | 5-8 | mg/L | Related to the process, not a key indicator for purity judgment |
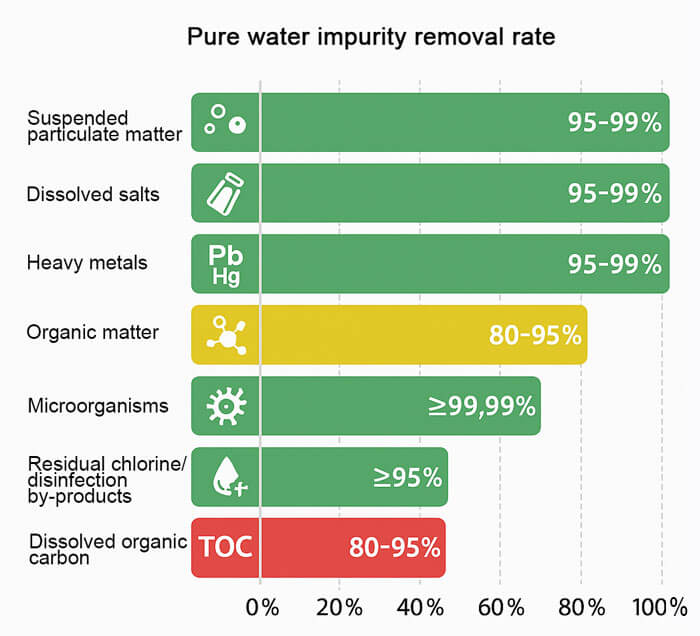
While both aim for high purity, purified water retains a certain amount of volatile substances and trace minerals during the filtration process. Its Total Dissolved Solids (TDS) value typically ranges between 10–30 ppm. This balance allows purified water to maintain a high level of cleanliness while offering a smoother, more pleasant taste, making it better suited for daily drinking.
Distilled Water vs Purified Water: What's the Difference?
Preparation
The method used to produce pure water depends on its specific application and required standards. Typical water purification techniques include ion exchange, reverse osmosis, nanofiltration, and distillation.
Ion exchange: Municipal tap water or groundwater is typically used as the source water. The process begins with filtration to remove larger particles and impurities. Common equipment used in this process includes sand filters, activated carbon filters, and water softeners.
Reverse Osmosis (RO): RO systems function based on the reverse osmosis principle. By applying external pressure, water is forced through a semipermeable membrane that allows only water molecules to pass through, effectively removing dissolved salts and other contaminants.
Nanofiltration: Nanofiltration membranes possess selective permeability. They allow water and certain beneficial minerals to pass, while filtering out larger ions and pollutants. This results in purified water that retains some essential minerals.
The process flow of distilled water involves heating the raw water to its boiling point to produce steam, leaving behind non-volatile dissolved impurities (such as inorganic salts, heavy metals, organic compounds, and microorganisms) in the evaporator. The steam is then directed into a condenser where it is cooled and transformed back into liquid water. Throughout this process, the water undergoes a physical change of evaporation and condensation, and purity can typically be enhanced by using multi-stage distillation.
| Comparison of purified water and distilled water processes | ||
|---|---|---|
| Item | Purified water | Distilled water |
| Process method | Membrane filtration, adsorption, ultraviolet sterilization, etc. | Heating evaporation + steam condensation |
| Removal efficiency | High (dissolved salts, organic matter, microorganisms) | Extremely high (almost all impurities) |
| Energy consumption | Low (mainly power consumption) | High (requires heating evaporation) |
| Equipment investment | Medium, complex structure | High, requires high temperature and high pressure corrosion-resistant equipment |
| Water production rate | Fast, suitable for large-scale continuous water production | Slow, limited output |
| Applications | Drinking, food, industrial cleaning | Laboratory analysis, pharmaceutical injection, chemical reaction |
Water quality
The electrical conductivity of purified water typically ranges between 10 to 100 μS/cm, mainly depending on the purification technology used. Although most ions have been removed, trace amounts of sodium ions, calcium ions, or silicate may still be present. Therefore, the TDS (Total Dissolved Solids) content of purified water generally ranges from 10 to 50 mg/L.
The electrical conductivity of distilled water usually ranges from 1 to 10 μS/cm. If multiple distillation processes or quartz distillation apparatus are used, it can be further reduced to <1 μS/cm. The distillation process effectively separates water from impurities, leaving almost no conductive ions. The TDS of distilled water can be as low as 1 to 5 mg/L, or even close to 0 mg/L.
Application
Purified water applications
- Drinking water: used as a safe and clean source of hydration, free from impurities.
- Medical and pharmaceutical: essential for the preparation of medications and as a solvent in medical applications.
- Cosmetic manufacturing: used as a base ingredient in cosmetics and skincare products to ensure safety and cleanliness.
- Food processing: utilized for rinsing, cleaning, and preparation of food products, ensuring hygiene standards.
- Laboratories: acts as a solvent for various laboratory procedures, ensuring the integrity of results.
- Industrial applications: used in cooling systems and other industrial processes where high purity is required to prevent damage to equipment.
Distilled water applications
- Medical equipment: used in autoclaves, humidifiers, and CPAP machines to prevent the buildup of minerals and contaminants.
- Laboratory applications: ideal for experiments requiring high-purity water, as it’s free of ions, minerals, and contaminants.
- Automotive cooling systems: used in car radiators and batteries to avoid scaling or mineral deposits.
- Aquariums: helps maintain a controlled and safe environment by providing water free of impurities.
- Steam irons: prevents mineral buildup and ensures efficient operation of steam irons.
- Electronics manufacturing: required in the production of electronic components to avoid contamination and ensure accuracy in delicate processes.
Video from @MedicalCentric
Distilled Water vs Purified Water: Which is best?
Both are safe to drink. Both distilled water and purified water undergo strict standards in their water quality treatment processes and meet the safety requirements for drinking water. Therefore, from a safety perspective, both can be consumed. However, their characteristics make them more suitable for long-term consumption in different ways.
The only real difference between distilled water and purified water is that purified water is treated with reverse osmosis (RO) filters or other methods to remove impurities and turbidity, while distilled water is subjected to heating treatment, which removes almost everything except hydrogen and oxygen.
In any case, both types of water are safe to drink. However, purified water has a better taste because it retains some minerals. Additionally, the minerals left in purified water can provide some nutritional support.
How to test water quality?
Although both distilled water and purified water are highly purified, their purification processes and uses differ, which requires the use of various water quality sensors to achieve precise assessment.
- Conductivity sensors can quickly determine the concentration of dissolved ions in the water, reflecting the overall purity level.
- TDS sensors are used to measure the total dissolved solids (TDS) content, making them suitable for monitoring purified water treatment.
- Resistivity sensors are more suitable for high-precision evaluation of ultra-pure water, such as distilled water.
- pH sensors monitor changes in the water’s acidity or alkalinity, helping to determine the presence of dissolved gases or system contamination.
For the purification process of purified water, free chlorine sensors are used to detect disinfectant residue before and after reverse osmosis. If used for drinking water, additional testing with heavy metal detection modules and TOC (Total Organic Carbon) analyzers is necessary to further confirm the purification effect.
By integrating with an online water quality monitoring platform, these sensors enable centralized data management and support features like alerts, report exports, and remote operations. By deploying a multi-parameter water quality monitoring system like those from Renke, a comprehensive water quality monitoring system can be established—from raw water treatment to end-use water, ensuring high-standard industry water safety.