What is conductivity in water?
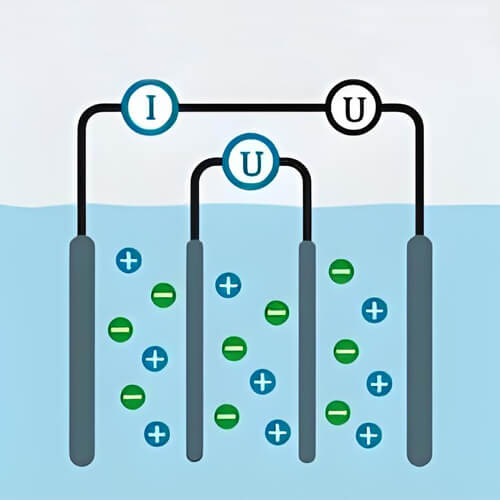
Conductivity in water is the ability of water to conduct electricity. It is primarily related to the amount and type of electrolytes dissolved in the water, and is secondarily affected by factors such as temperature and pressure. Electrolytes are compounds that break down into free-moving ions when dissolved in water, and these ions are capable of conducting electricity. In water, electrolytes break down into positive and negative ions. Therefore, as the type and amount of electrolytes increase, the more conductive the water becomes.
Factors affecting the magnitude of conductivity in water. The conductivity of natural water is related to the total number of ions and types of ions in the water, as well as to temperature and pressure, but the effect of pressure is relatively small. As long as field measurements are not made in deep water, the effect of pressure can be ignored.
Why is conductivity in water important?
Conductivity in water is one of the important parameters for water quality monitoring. The conductivity of water is measured by the degree of penetration of electricity in the water. It is of no use to try to pass electricity to pure water because pure water contains almost no ions that can conduct electricity. The presence of a large number of ions permits greater water conductivity. Seawater, however, contains a large number of conductive ions, and water conductivity measurements are often used as an indicator of how healthy the oceans and the organisms in them remain.
In addition to studying the health of seawater, it is also possible to monitor the salinity in seawater. The salinity of water is the amount of dissolved salt in the water. Conductivity and salinity are interrelated because they correlate well. The amount of salt dissolved in water cannot be measured directly, but conductivity in water can easily be measured separately and can be used to identify the total dissolved solids and salinity concentration in water, thus monitoring the living environment of aquatic organisms. The salinity of dissolved water in nature affects the dissolved oxygen content.
Conductivity values can also be used as an early indicator of changes in water quality. Most water maintains a fairly constant conductivity, which can be used as a standard for comparison with future measurements. Significant changes in water quality, whether due to natural flooding, evaporation or man-made pollution, can be extremely damaging. Preventing problems with water quality requires frequent testing of the conductivity in water.
High or low conductivity in water
What causes high conductivity in water?
Presence of ions: Water has conductivity when there are charged and free-moving ions in the water. The lack of conductivity in pure water is the lack of a variety of free-moving ions, since ions are able to move in response to an electric field, which conducts an electric current. Gases in the air can also change the conductivity in water when they come into contact with it. For example, carbon dioxide in the air dissolved in water will form carbonic acid and dissociate hydrogen ions and carbonate ions, these ions improve the conductivity in water.
More ions: In two cups of pure water of the same volume, add different amounts of electrolytes will find that the more electrolytes the water, the more conductive it is. This is because more electrolyte means that the number of free-moving ions in the water is greater, from to the ability to conduct an electric current.
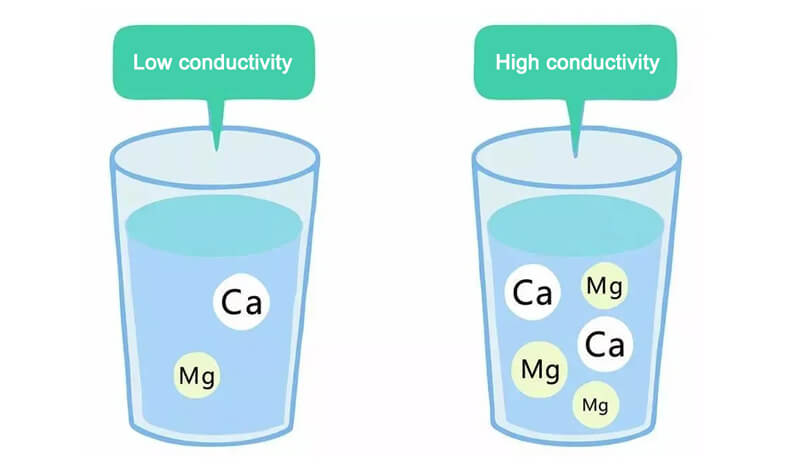
Multiple types of ions: Different types of ions have different conductivities. Some ions are able to conduct electricity more efficiently, so if more than one type of ion is present in the water, the conductivity may be higher. When water is dissolved with salt or other electrolytes, these dissociate into ions, which enhance the conductivity in water. As a result, bodies of water with a high salt content (e.g., seawater) tend to have a higher conductivity, while pure water has a relatively low conductivity due to its very low concentration of ions.
Temperature: the conductivity in water usually increases with increasing temperature. This is because higher temperatures increase the rate of movement of ions in the water, which increases the conductivity. For every 1 degree Celsius increase in water temperature, the conductivity value increases by 2-4%. Temperature affects conductivity by increasing ion mobility and the solubility of many salts and minerals.
What does high conductivity in water indicate?
The amount of dissolved salts, pollutants and minerals can be reflected by monitoring the value of conductivity in water. The higher the conductivity, the higher the type and concentration of ions in the water. In the natural environment, on the one hand, rainwater carries dissolved minerals from the soil into the river, resulting in higher conductivity in water. On the other hand, agricultural pollution, industrial effluents and other human activities that increase pollutants in the water can lead to an increase in the conductivity of the water. However, high conductivity in water does not mean poor water quality; it may also be the result of high amounts of dissolved non-toxic and harmless electrolytes.
Is lower conductivity in water better?
Conductivity in water is a physical parameter related to the dissolved substances in water and indicates the ability of water to conduct electricity. The conductivity of pure water is very low, usually around 50 microsiemens (µS). However, when water contains dissolved ions (e.g., sodium chloride, calcium, magnesium, etc.), the conductivity increases.
In nature, the conductivity of natural water varies greatly depending on the soil and geographical location. The electrolytes of river and lake water are typically between 100 μS and 1000 μS. Industrial water, seawater, or water from other highly saline areas can have a conductivity of thousands of Siemens (S).
Objectively speaking, the conductivity in water does not determine the quality of water, but rather serves as an indicator to help determine the amount of dissolved substances in the water. For example, adding some sugar and salt to water will result in a higher conductivity, but such water is not harmful to the body. Similarly, water with a low conductivity does not necessarily mean that it does not contain toxic substances. Evaluating water quality requires a combination of other physical and chemical indicators and testing equipment, such as PH sensors, turbidity sensors, etc.
Applications for conductivity measurement
Assessing the purity of water
Measuring the conductivity in water is one of the most important means of assessing the purity of water. Typically, pure water has a very low conductivity because it contains almost no ions. On the contrary, water containing more ions (e.g. seawater, salt water or contaminated water) has a higher conductivity. Therefore, by measuring the conductivity, we can get a general idea of the purity of the water.
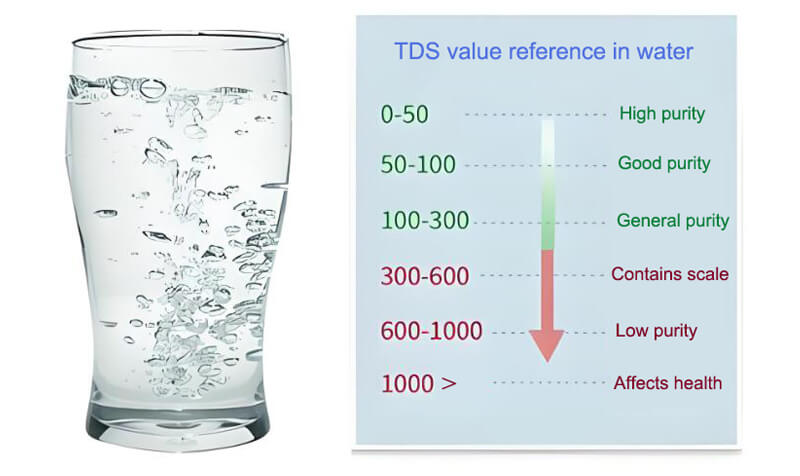
Detecting salt in water
Conductivity is also an effective method of detecting salt content in water. The dissociation of salt (mainly sodium chloride) in water produces large amounts of sodium and chloride ions, which significantly increase the conductivity in water. This is important for desalination, food processing, agricultural irrigation and other fields.
Monitoring industrial water
In industry, the water quality of cooling water, boiler water and various process water is monitored by measuring conductivity. Based on real-time changes in conductivity values, managers can detect changes in water quality in time to take appropriate measures to protect equipment from corrosion and scaling. This is essential to ensure the continuity and safety of industrial production.
Environmental monitoring
In natural environmental protection, measuring the value of conductivity can assess the status of water quality in rivers, oceans, lakes and so on. Through long-term monitoring of conductivity changes in these waters, the presence of pollution problems can be detected and pollutants can be analyzed in a timely manner, thus providing valuable data support for environmental protection and governance.
How to measure conductivity in water?
1. Conductivity electrode
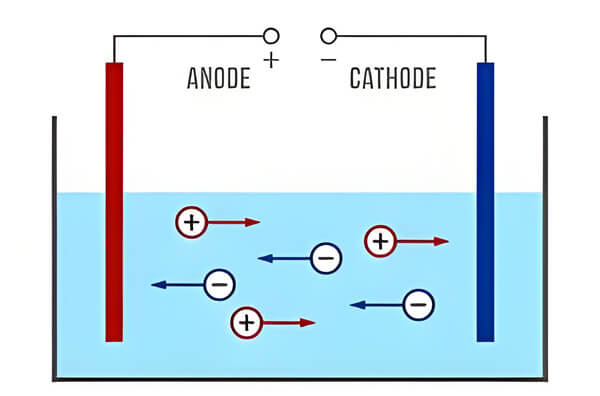
The conductivity electrode method is a common measurement method. It works on the principle that an electrode inserted into the water reacts with the ions to produce When the electrode is immersed in the water, the ions in the water react with the electrode to produce a weak electric current. The magnitude of the current is positively correlated with the concentration of ions in the water, and thus the conductivity is calculated. The conductivity electrode method is commonly used for real-time measurements in the field. However, the process of electrode reaction is easily affected by water temperature and other impurities, resulting in errors in the data. Therefore regular calibration and maintenance is required during use.
2. Conductivity test paper
The conductivity test paper method is a very simple method of measurement. This method uses a conductivity paper similar to PH paper and gives a rough reading of the conductivity value by comparing the color of the paper to a standard color card. Although the accuracy of this method is relatively low, it is simple to operate and is suitable for emergency monitoring in the field or for home use. Since the test paper has a limited life span and needs to be purchased and replaced periodically, it is not suitable for sites that require long-term conductivity monitoring.
3. Water conductivity sensor
Conductivity sensors are a highly accurate, automated measurement method. This method uses a high-precision conductivity sensor to continuously monitor the conductivity in water through automated equipment. The sensor can be used in conjunction with a control system. This method is highly accurate and stable, and is suitable for monitoring in a variety of environments. Wastewater treatment systems usually use the conductivity sensor in conjunction with other water quality sensors such as the PH sensor, dissolved oxygen sensor, and turbidity sensor.
4. Remote sensing measurement
Remote sensing measurement is a non-contact method of measuring water quality through remote sensing equipment carried by satellites or airplanes. This method can obtain a large range of water quality data and is fast in measurement. However, the accuracy of the remote sensing measurement method tends to be low due to the influence of factors such as water transparency and water color. In addition, the cost and maintenance of remote sensing equipment is relatively high, so the scope of use is relatively small.