What is ORP in water?
ORP is Oxidation-Reduction Potential. It is an index that measures a solution’s ability to gain or lose electrons, indicating whether the solution tends to be oxidizing or reducing.
In liquid environments, oxidation-reduction potential is defined as the voltage difference between a redox (indicating) electrode and a reference electrode, providing a comprehensive measure of the redox condition of the solution.
- A positive ORP value indicates that the water environment tends toward oxidation. The higher the value, the stronger the water’s oxidizing ability, meaning it is more likely to capture electrons from other substances. For example, water containing chlorine, ozone, or a high concentration of dissolved oxygen typically exhibits a high positive ORP value.
- A negative ORP value indicates that the water environment tends toward reduction. The lower the value, the stronger the reducing ability of the water, meaning it is more likely to donate electrons to other substances. For instance, under oxygen-deficient or anaerobic conditions, due to the presence of reducing agents such as hydrogen sulfide, ferrous ions, or high levels of organic matter, the water usually displays a negative ORP value.
The range of oxidation-reduction potential in water varies depending on the application. Generally, the ORP value of drinking water is usually between +200mV and +600mV, and that of swimming pool or disinfectant water can reach above +650mV, which has a strong bactericidal effect. In aquaculture, the appropriate value is usually +250mV~+400mV, while in sewage, the ORP value may be lower than 0mV or even reach below -200mV.
How to measure ORP?
Oxidation-Reduction Potential is typically measured in millivolts (mV) using specialized ORP sensors or probes. To take a reading, the probe is submerged in the liquid—this can be done using a portable device or an integrated system.
These sensors work by detecting voltage through electrodes, allowing for quick and cost-effective readings across a wide range of applications. A higher oxidation-reduction potential value generally indicates a cleaner, more disinfected water source.
ORP probes are highly safe and easy to use. In water quality monitoring applications, they are commonly used alongside pH sensors, dissolved oxygen sensors, conductivity (EC) sensors, chlorine sensors and so on. If you’re unsure about how to choose the right water quality sensor, feel free to contact us. We will provide the optimal water monitoring solution tailored to your specific requirements and application environment.
How does an ORP sensor work?
Oxidation-Reduction Potential sensors usually consist of a measuring electrode (composed of precious metals such as platinum or gold) and a reference electrode (such as Ag/AgCl). During use, the measuring electrode responds to the exchange of electrons between oxidized and reduced species in the solution, thereby generating a potential difference relative to the reference electrode. This voltage difference is the ORP reading.
It is worth noting that the ORP electrode measures the combined results of all redox reactions in the solution, not just the redox potential of a single substance. Therefore, the oxidation-reduction potential value can reflect the overall redox state of the solution.
Factors that affect ORP
The Oxidation-Reduction Potential value in water is the result of the combined effect of multiple factors. These factors can be divided into three categories: chemical (pH, dissolved oxygen, oxidants and reductants), physical (temperature, ultraviolet light) and biological (microorganism types and activities). They directly participate in the redox reaction or change the reaction conditions.
1. pH
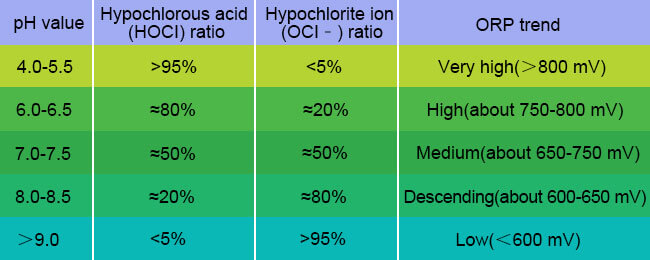
The change of Oxidation-Reduction Potential in water is affected by many factors, among which the change of pH is one of the core factors. This is mainly because redox reactions often involve the participation of hydrogen ions (H⁺), and pH changes directly affect the thermodynamic drive of the electron transfer process. When the pH level increases, the ORP value will decrease accordingly; conversely, when the pH value decreases, the ORP value will increase.
Take the use of chlorine disinfection in swimming pools as an example. When chlorine or hypochlorite is dissolved in water, an equilibrium system of hypochlorous acid (HOCl) and hypochlorite ions (OCl⁻) is formed. Among them, HOCl is a highly efficient oxidant and disinfectant, while OCl⁻ has a much weaker oxidizing ability, and its bactericidal efficiency is only 1/80 to 1/300 of HOCl. At this time, the pH value of the water will affect this equilibrium system.
(Effect of pH on Available Chlorine Form and ORP)
When the pH value is in the range of 7.0-7.5, most of the available chlorine in the water exists in the form of strong HOCl. This makes the water have strong oxidation and disinfection capabilities, and the Oxidation-Reduction Potential value is also high.
When the pH value rises above 8.0, a large amount of HOCl will dissociate into weak OCl⁻. Although the total residual chlorine (ppm) measured by the DPD method may not change at this time, the actual disinfection ability of the water has dropped significantly, which will be accurately reflected by the significant decrease in the Oxidation-Reduction Potential value.
2. Dissolved oxygen
Oxygen is a typical strong oxidant. When it is dissolved in water, it can participate in a variety of redox reactions. The more oxygen dissolved in water, the more it can promote microbial metabolism and the decomposition of organic matter. At this time, the electron flow in the water is transferred from organic matter to oxygen molecules, and the ORP value also increases. According to the EPA water quality report, when DO increases from 2 mg/L to 8 mg/L, ORP increases from about 300 mV to 500 mV.
Increasing the dissolved oxygen concentration by aeration will significantly increase the ORP value of water, creating favorable conditions for the activities of aerobic microorganisms (such as organic matter degradation and nitrification). On the contrary, when the rate at which the organic matter and other reducing agents in the water consume dissolved oxygen exceeds its replenishment rate, the DO concentration decreases, and the ORP also decreases accordingly, and the water gradually changes from an aerobic state to an anoxic or even anaerobic state.
3. Oxidants and reductants
Oxidants are substances that can accept electrons. When their concentration increases, water will have a stronger ability to attract electrons, thereby increasing the Oxidation-Reduction Potential value. Common oxidants in water include hypochlorous acid (HOCl), ozone (O₃), chlorine (Cl₂), permanganate (MnO₄⁻), etc. The higher the concentration of oxidants in water, the greater the ORP value is, showing a strong oxidizing environment. For example, in swimming pool water, the ORP value is higher than +650 mV, indicating that the sterilization and disinfection effect is good.
Reductants are substances that can release electrons. When their concentration increases, the number of electrons in the water increases, counteracting the effect of oxidants and thus reducing the Oxidation-Reduction Potential value. Common reductants in water include organic matter, sulfide (S²⁻), nitrite (NO₂⁻), ferrous ions (Fe²⁺), hydrogen (H₂), etc. When the content of reductants in water is high, the ORP value is significantly reduced, and the water environment is reducing, which is common in anaerobic areas of sewage or polluted water bodies.
4. Temperature
Increased temperature accelerates the rate of almost all chemical and biological reactions in water. This changes the consumption and generation rate of oxidants and reductants, thereby changing the Oxidation-Reduction Potential value. In addition, as the water temperature increases, the lower the saturated dissolved oxygen concentration, the more changes in Oxidation-Reduction Potential will occur.
The effect of temperature on Oxidation-Reduction Potential is clear, and the given value of the ORP calibration standard solution changes with temperature. For example, the standard value of the commonly used Zobell standard solution is 228 mV at 25°C, and 241 mV at 15°C. Therefore, it is worth noting that, similar to pH sensors, when you choose ORP sensors, you should also choose devices with temperature compensation function to ensure accurate and effective measurement values.
5. Ultraviolet light
Ultraviolet (UV) light from sunlight is a powerful energy source that can directly decompose certain oxidants in water, particularly hypochlorous acid (HOCl) and ozone (O₃). This photolysis reduces the concentration of oxidants, resulting in a decrease in the Oxidation-Reduction Potential.
The effects of UV light are most pronounced in outdoor pools and open water, where it causes the ORP to exhibit a typical diurnal fluctuation pattern. Typically, ORP values are at their lowest point in the afternoon when sunlight is strongest, and rise again during the night.
6. Microorganisms
In various water environments, microbial activity is an important biological factor driving ORP changes. Microorganisms continuously participate in redox reactions through their metabolic activities, changing the electron concentration in water, thereby affecting the change in Oxidation-Reduction Potential.
In addition to the metabolic stage, the type of microorganisms will also affect the change of ORP in water. Different bacterial communities have different enzyme systems, electron transport chain structures and substrate preferences, so they contribute differently to Oxidation-Reduction Potential under the same environment.
For example, aerobic nitrifying bacteria (such as Nitrosomonas) are suitable for high oxygen environments of +300~+500 mV, using oxygen to oxidize ammonia nitrogen. Denitrifying bacteria (such as Pseudomonas) prefer low oxygen environments of +50~+250 mV to reduce nitrates to nitrogen gas. Iron-reducing bacteria are active in reducing environments of -50~+100 mV, using Fe³⁺ as electron acceptors. Sulfate-reducing bacteria and methanogens are suitable for growing in strong reducing environments of -100 mV and -200 mV, respectively, producing hydrogen sulfide and methane. Changes in ORP values can also reflect the types of microorganisms in the water and their metabolic processes.